
The Nathanson Lab focuses on malignant brain tumors, in particular glioblastoma (GBM) – one of the most lethal of all cancers. We take an interdisciplinary approach to understand the biology of brain cancer and to develop innovative therapeutics to ultimately improve on patient outcomes
RESEARCH
FUNCTIONAL BIOLOGY OF BRAIN CANCER
Cancers, including malignant glioma, have hijacked and rewired many normal cellular functional processes in order to grow and survive. These cancer “hallmarks” – as defined by Hanahan and Weinberg (Cell 2011) – include resisting cell death and deregulated metabolism. Notably, reprogramming of these functional pathways in cancers is a direct consequence of aberrant oncogenic signaling emanating from the loss of tumor suppressors and/or mutations in oncogenic drivers. Accordingly, our lab has hypothesized that investigating the interplay between oncogenic signaling and metabolism/cell death pathways can reveal novel therapeutic vulnerabilities.
DRUG DISCOVERY AND DEVELOPMEnt of brain-penetrant therapies
The effective treatment of brain tumors requires that drugs get into the brain to target the tumor. However, the vast majority of FDA approved drugs do not cross the blood brain barrier (BBB) – a physical barrier that is highly selective for what can cross into the brain. Through collaborations with Dr. Michael Jung (Distinguished Professor of Chemistry, Co-Inventor of two FDA approved drugs for cancer), we have established a drug development program that is focused on synthesizing and testing new, potent and brain-penetrant drugs that specifically target malignant glioma cells.
Developing Innovative Pre-clinical Models of brain tumors
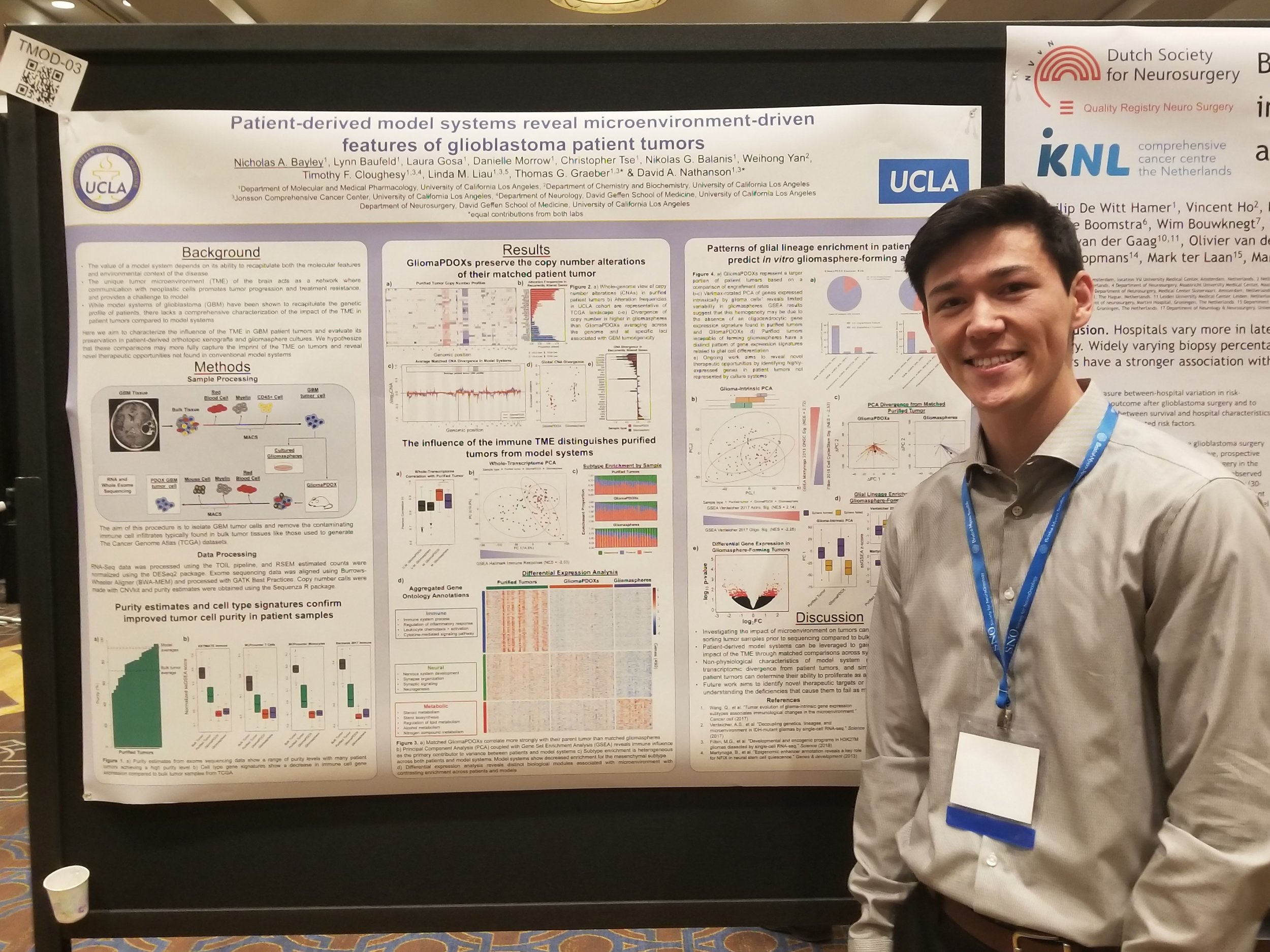
The translation of therapeutic strategies for treating several malignancies have been hampered by the lack of robust pre-clinical models that accurately reflect the human disease. This is particularly relevant in malignant gliomas, where the genetic and functional properties of conventional cell lines show dramatic divergence from the original patient samples. This has major implications for understanding the biology of these tumors and the development of new therapies. To address this, we have created a novel patient-derived, orthotopic glioma xenograft library (termed GliomaPDOX). This library captures the vast molecular diversity of GBM.
MOLECULAR Diagnostics OF BRAIN TUMORS
Although all gliomas originate in the brain, the molecular alterations are often unique between each patient’s tumor. These distinctions can have profound implications for diagnosis and therapeutic response. Our laboratory uses next generation sequencing – including RNAseq and exome sequencing – of patient and GliomaPDOX tumors to precisely define the molecular abnormalities that are driving each glioma. To complement this, we also employ non-invasive molecular imaging, such as positron emission tomography (PET), to examine how imaging can predict tumor response to therapeutics targeting specific molecular alterations in glioma patients and models.
FEATURED PUBLICATIONS
Nathanson Lab poster presentation at EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium in Barcelona, demonstrating ERAS-801 activity in preclinical models of brain metastasis lung cancer.
CDKN2A deletion remodels lipid metabolism to prime glioblastoma for ferroptosis
Malignant tumors exhibit heterogeneous metabolic reprogramming, hindering the identification of translatable vulnerabilities for metabolism-targeted therapy. How molecular alterations in tumors promote metabolic diversity and distinct targetable dependencies remains poorly defined…
Development of a Potent Brain-Penetrant EGFR Tyrosine Kinase Inhibitor against Malignant Brain Tumors.
The epidermal growth factor receptor (EGFR) is genetically altered in nearly 60% of glioblastoma tumors; however, tyrosine kinase inhibitors (TKIs) against EGFR have failed to show efficacy for patients with these lethal brain tumors…
Cytoplasmic p53 couples oncogene-driven glucose metabolism to apoptosis and is a therapeutic target in glioblastoma.
Cross-talk among oncogenic signaling and metabolic pathways may create opportunities for new therapeutic strategies in cancer….
Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA
Single-cell analyses of patient-derived models and clinical samples from glioblastoma patients treated with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) demonstrate that tumor cells reversibly up-regulate or suppress mutant EGFR expression…
Elizabeth Fernandez,
PhD Candidate
Nick Bayley,
PhD Candidate
Jenna Minami,
PhD Candidate
Quincy Okobi,
PhD Candidate
Marissa Pioso,
PhD Candidate
Jennifer Salinas,
PhD Candidate
Dimitri Cadet,
MD-PhD Candidate
Cassidy Andrasz, PhD Candidate
Armon Goshayeshi,
PhD Candidate
Research Staff
Christopher Tse,
Program Manager
Michael Vigman,
Drug Discovery
Allie Yoon,
Drug Discovery
Amir Borujerdpur, Bioinformatics
Raul Davila,
Model System Development
Ronald Bernal,
Model System Development
Max Johnson,
in vivo Drug Development
Ivan Khoroz,
in vivo Drug Development
Rashmika Kulshrestha,
in vivo Drug Development
Undergraduate Research Assistants
Melody Jiang, Undergraduate Student Researcher
Alanna Lemp, Undergraduate Student Researcher
Marissa Li, Undergraduate Student Researcher
Toby Harris, Undergraduate Student Researcher
Molly McAndrew,
Undergraduate Student Researcher
Sarah Lee,
Undergraduate Student Researcher
Karim Gonzalez,
Undergraduate Student Researcher
Christian Lee,
Undergraduate Student Researcher
Aliya Sabater,
Undergraduate Student Researcher
LAB NEWS







-
Congratulations Marissa Pioso, joint graduate student at @NathansonLab and @UCLANsgy Prins Lab, for advancing to ca… https://t.co/2IzBkkfSGl
-
RT @Leia_N_MD: @UCLANeurology Neuro-Onc team taking a break with the people who are developing the treatments to fight #braintumor… https://t.co/58ADdA55j1
OPEN POSITIONS
Postdoctoral Researcher
Open position in translational brain cancer research focusing on cell signaling and apoptosis.
CONTACT
Want to learn more or get involved? Contact Us!
UCLA CHS 23-234
650 Charles E Young Dr South
Los Angeles, CA 90036
+1 (310) 825-6006

Create
LASTING
IMPACT
Every dollar moves us closer to the end of brain cancer.
Your donation to lab research can change lives.











































